Beyond bulk averaging: why single-cell matters
Biological research has relied on “bulk” analysis where molecular data is collected from millions of cells simultaneously. While powerful, this approach inherently averages the data, effectively masking the critical cell-to-cell variations that are fundamental to understanding disease progression, drug response, and developmental processes. This averaging can obscure the identification of rare subclones or subtle molecular changes within the cell population, leading to an incomplete picture of the sample. The average readout from conventional bulk sequencing, for instance, often misses the underlying genetic diversity across cell populations, providing an inferred or guessed clonal architecture rather than a precise measurement.
The advent of single-cell analysis has enabled researchers to probe the biological complexities in a cell population at the single-cell level. This approach could analyze DNA, RNA, protein, or a mix of the three biomolecules within individual cells, revealing a better understanding of CNV, zygosity, hotspot mutations in driver & resistance genes, RNA fusions, gene expression, V(D)J clonotyping, surface immunophenotyping, and more (Table 1, Figure 1).
Table 1: Single-cell analysis uncovers biological complexities of a sample at a single-cell level
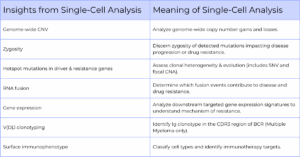
Single-cell sequencing examines the nucleic acid sequence information from individual cells using next-generation sequencing (NGS) technologies to understand genomic differences and better insights of the function of an individual cell within its microenvironment. This capability to isolate, analyze, and profile individual cells is crucial for understanding cellular heterogeneity, gene expression, and metabolic activities.
Building on this, the concept of “multi-omics” emerged, representing a biological analysis approach that integrates data from multiple “omes,” such as the genome, transcriptome, and proteome. By combining data from genomics, transcriptomics, and proteomics, researchers can achieve a more comprehensive understanding of molecular changes contributing to normal cellular development, cellular responses when a population is stressed or exposed to a drug molecule, and even therapy development and patient stratification for diseases like multiple myeloma.
While single-cell omics have advanced significantly, separately developed methods limit the ability to accurately analyze how different biomolecular layers regulate each other within a single cell. To directly analyze the mechanisms by which genetic mutations regulate gene expression and protein translation in individual cells, the genome, transcriptome, and proteome need to be simultaneously analyzed within that single cell. This direct, single-cell measurement of thousands of cells within a sample population is foundational for understanding complex regulatory pathways and disease mechanisms, moving beyond observational studies to clinical applications such as patient stratification and demonstrating persistent endpoints for therapeutics.
Understanding single-cell multi-omics
A single cell is the fundamental unit of biological function and disease, and in cell and gene therapies, it can be the drug product itself. Even within seemingly homogeneous tissues, individual cells exhibit unique molecular profiles and behaviors. Single-cell techniques are commonly used to study cell development, identify rare cell populations, and characterize cellular responses to stimuli. This allows for the identification of subtle but significant differences in gene expression, metabolic activity, and responses to stimuli that are critical for understanding diseases, especially where rare cell populations drive pathology, such as cancer cells or drug-resistant clones.
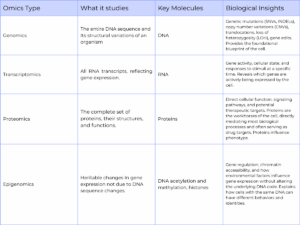
The “omics” landscape provides a multi-layered view of biology (Table 2), with each biological layer offering distinct but complementary information:
- Genomics focuses on the structure, function, evolution, mapping, and editing of an organism’s DNA. It is the foundational layer, revealing genetic variations such as single nucleotide variants (SNVs), copy number variants (CNVs), insertions-deletions (INDELs), loss of heterozygosity (LOH), and translocations.
- Transcriptomics involves the study of the complete set of RNA transcripts produced by the genome, reflecting gene expression and cellular activity at a given time. Single-cell RNA sequencing (scRNA-seq) has become a common technique for unraveling the heterogeneity and complexity of RNA transcripts within individual cells.
- Proteomics evaluates protein expression for a better understanding of cellular function and prediction of therapeutic responses. Proteins are the primary effectors of cellular processes, and their expression levels offer direct insights into cellular function influencing a cell’s phenotype.
- Epigenomics examines heritable changes in gene expression activity caused by factors other than DNA changes, such as DNA methylation or chromatin accessibility. Profiling chromatin accessibility can identify candidate regulatory genomic regions in a tissue or cell type.
Multi-omics integrates data from these different biological layers to provide a more comprehensive understanding of biomolecular changes contributing to normal development, cellular response, and disease. This approach offers a more complete way to understand the contribution of genetic variants to biology, disease, and their mechanism of action.
Tri-omics can refer to the simultaneous analysis of the genome, transcriptome, and proteome within a single cell. This enables analysis of genomic copy number variations (CNVs), gene expression, and phenotype at the single cell level, revealing their unique genetics, transcriptomics, and proteomics within a heterogeneous population sampled from thousands of cells. The integration of these biological layers allows researchers to move beyond observing individual molecular events to understanding the complex regulatory pathways and causal relationships between genetic mutations, gene expression, and protein translation.
Table 2: Key “omics” and their value in single-cell analysis
Values of performing single-cell multi-omics vs single-omics approaches
Performing single-cell multi-omics offers significant advantages over sequential single-omics approaches by capturing genomic, transcriptomic, and proteomic information from the same cell simultaneously, enabling direct correlations between biomolecular layers and reducing the amount of sample needed for each -omics assessment, processing time, and even cost.
How can single-cell multi-omics determine cellular heterogeneity?
Cellular heterogeneity is a critical factor in understanding diseases where different subclones can drive resistance or metastasis, particularly in complex diseases like acute myeloid leukemia (AML), multiple myeloma (MM), solid tumor cancers, and others. The average readout from conventional bulk sequencing misses the underlying genetic diversity across cell populations, leading to inferences rather than precise measurements of clonal heterogeneity. Single-cell multi-omics directly measures this heterogeneity, allowing for the identification of subtle but significant differences in gene expression, metabolic activity, and responses to stimuli that are critical for understanding cancer and other diseases, especially where rare cell populations drive pathology.
Can single-cell multi-omics link correlation to causation?
The ability to simultaneously measure multiple biomolecular layers within the same cell allows researchers to directly observe, for example, how a specific DNA mutation (genomics) impacts gene expression (transcriptomics) and subsequently protein translation (proteomics). This moves beyond statistical correlations derived from separate experiments to direct, unified datasets that provide deeper understanding into disease mechanisms. To accurately analyze the mechanism by which the genome, transcriptome, and proteome regulate each other, these omic methods need to be performed in the same single-cell. This direct simultaneous assessment within a single cell is crucial for understanding true biological mechanisms and causal relationships, representing a better level of understanding than mere correlation. Furthermore, combining multiple assays in a single integrated workflow can reduce costs and speed up timelines time, making drug development pipelines more efficient and drugs that can be affordable.
Identify rare cell subclones that drive disease and resistance using single-cell multi-omics
Single-cell multi-omics excels at detecting and characterizing rare cell populations that are often missed by bulk analysis but can be disproportionately important in disease pathology or therapeutic response. For instance, detecting rare subclones down to 0.1% of the population that may be missed by conventional bulk sequencing. Single-cell multi-omics analysis enables the precise detection and characterization of these rare subclones, which helps with understanding disease relapse, identifying minimal residual disease (MRD), and developing therapies that target these elusive rare subclones, leading to more durable patient responses. Leveraging single-cell multi-omics can also support patient stratification, helping determine the most effective, personalized therapy regimen for each individual.
How does genotype-to-phenotype single-cell multi-omics data help with developing better therapies?
Integration of genomic, transcriptomic, and proteomic data from individual cells gives researchers a holistic view of cellular function and dysfunction. This approach bridges the gap between genetic mutations and observable cellular traits, providing a “true picture from genotype to phenotype.” It enables detailed analysis of single-nucleotide variants (SNVs), insertions and deletions (INDELs), focal and genome-wide copy number variants (CNVs), loss of heterozygosity (LOH), chromosomal translocations, and surface protein expression, all within the same cell. By capturing these multiple biomolecular layers simultaneously, single-cell multi-omics allows scientists to directly link genetic changes to gene and protein expression, revealing how mutations influence cellular behavior in diseases. This comprehensive understanding helps uncover disease mechanisms, identify therapeutic targets, and guide the development of more precise and effective treatments, all informed by insights at the single-cell level.
How to incorporate single-cell multi-omics in therapy development
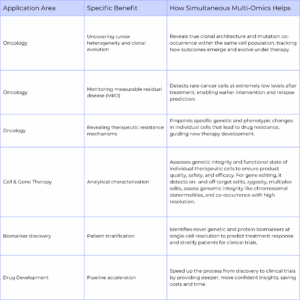
For treating diseases and during therapy development, scientists can get the granularity they need at the single-cell level to make the right decisions during development and treatment of patients when using single-cell multi-omics assays (Table 3). In oncology, scientists can uncover rare or disease-driving subclones, map tumor heterogeneity, and track clonal evolution, enabling earlier detection of measurable residual disease and revealing mechanisms of therapeutic resistance. For cell and gene therapies, it ensures the safety and efficacy of engineered cells by profiling genetic modifications and functional protein expressions at single-cell resolution. This higher resolution data also drives biomarker discovery and patient stratification, identifying predictive signatures that guide clinical trial enrollment and personalized treatment strategies. Even across drug molecule development, single-cell multi-omics data can speed development, reduce clinical trial attrition, and inform the design of more effective therapies, ultimately bringing treatments to patients faster.
What value does single-cell multi-omics bring to the field of oncology
Single-cell multi-omics improves cancer research by providing a better view of tumor biology:
- Understanding tumor heterogeneity and clonal evolution allows for mapping the complex clonal hierarchy and clonal architecture of tumors and tracking how different subclones emerge and evolve under selective pressures, such as therapies. It reveals the true clonal architecture in a tumor sample without the assumptions confounded by bulk sequencing and can identify true mutation co-occurrence in clonal populations. This is critical for understanding the evolutionary pathways of resistance and relapse, guiding the development of dynamic treatment strategies.
- Monitoring measurable residual disease (MRD) for relapse prediction is the ability to detect extremely low levels of residual disease after treatment and is a strong predictor of relapse. Single-cell multi-omics offers the sensitivity required to detect these rare cancer cells, enabling more precise and earlier detection of MRD. This provides clinicians with the opportunity to intervene sooner, potentially improving patient outcomes and guiding individualized treatment strategies.
- Revealing mechanisms of therapeutic resistance through single-cell multi-omics analysis can pinpoint the specific genetic and phenotypic changes in individual cells that lead to drug resistance. By tracking molecular changes in individual cells over the course of treatment, researchers can identify the genetic alterations or phenotypic shifts that confer resistance, leading to the development of targeted therapies that overcome these resistances.
Ensuring safety and efficacy of cell and gene therapy using single-cell multi-omics
Rigorous characterization is paramount for the development of safe and efficacious cell therapies like CAR-T cells. Single-cell multi-omics enables simultaneous measurement of multiple genotypic and phenotypic biomolecule attributes in cell therapy products, providing the most comprehensive insights into therapeutic function and quality. This approach not only ensures thorough characterization of ex vivo cell products, supporting consistency, safety, and optimized manufacturing, but also allows for continuous monitoring of therapeutic cells after being administered, offering real-time insights into persistence, function, and patient response to this cell therapy.
Ensuring safety and efficacy is critical for gene therapies, particularly those using genome editing tools like CRISPR, which can introduce precise modifications to the genome. However, editing outcomes can vary widely between individual cells within a treated population, producing heterogeneity in zygosity, off-target effects, co-occurring multiplex edits, and chromosomal translocations. Traditional bulk analysis methods average signals across millions of cells, masking this important cell-to-cell variability and limiting the ability to fully assess therapeutic risk. Single-cell multi-omics overcomes these limitations by simultaneously measuring DNA, RNA, and protein within the same cell. For gene-edited therapies, this approach allows precise quantification of both on- and off-target edits, evaluation of multiplex editing efficiency, determination of zygosity, and detection of chromosomal abnormalities, all at single-cell resolution. By providing a complete, high-resolution view of editing outcomes, single-cell multi-omics equips developers with a powerful tool to optimize safety, monitor genome integrity, and guide the clinical translation of gene therapies.
Single-cell multi-omics for biomarker discovery and personalized therapy
Single-cell multi-omics enabling the discovery of biomarkers across genomic, transcriptomic, and proteomic layers. These biomarkers help stratify patients for clinical trials, predict therapeutic responses, and identify individuals at higher risk of adverse events or disease progression. By revealing patterns invisible to single-omics or bulk analyses, researchers can define disease subtypes and select patients most likely to benefit from specific therapies, reducing variability that could obscure trial outcomes.
How to speed up drug development using single-cell multi-omics
Single-cell multi-omics provides high-resolution data insights during drug development, enabling researchers to make informed decisions earlier like optimize molecules faster or pivot from failing strategies quicker. By simultaneously analyzing genomic, transcriptomic, and proteomic data from individual cells, developers can better understand mechanisms of action, identify potential safety concerns, and optimize drug candidate selection before IND submissions. This early, detailed knowledge helps reduce trial attrition, improve study design, and increase the likelihood of successful clinical trials.
In practice, single-cell multi-omics is being used across pharmaceutical and biotech development programs to improve efficiency at every stage, from discovery and preclinical studies to clinical trials. By leveraging single-cell multi-omics, companies can accelerate the development of their therapies while maintaining confidence in safety and efficacy, ultimately bringing effective treatments to patients quicker.
Table 3: Key clinical applications of single-cell multi-omics in therapy development
The future of precision medicine requires single-cell multi-omics
Single-cell multi-omics represents a transformative leap in biological research and therapy development. By moving beyond the limitations of bulk analysis and providing a holistic view of individual cells, single-cell multi-omics is changing our understanding of biology and diseases. It allows researchers to unravel the complexities of cell population heterogeneity, directly understanding the regulatory relationships between different biomoleculars, and pinpoint rare yet critical cell populations that drive disease and resistance.
Companies like Mission Bio are advancing our understanding in fields like oncology, enabling more precise monitoring of measurable residual disease, and revealing mechanisms of therapeutic resistance. Furthermore, they have been critical for the rigorous characterization of cell and gene therapies, facilitating biomarker discovery, and helping companies speed up their drug development pipelines. As a company of passionate people dedicated to solving complex biological problems, Mission Bio is providing the tools necessary to characterize diseases and advanced therapeutics at the single-cell level to empower next-generation therapies.
Are you developing a drug molecule or cell & gene therapy? Want to use a custom, fit-for-purpose single-cell multi-omics assay for your program that provides you the single-cell resolution needed that regulatory agencies will want in data packages? Let’s plan your project on a call. Schedule a call today.
Additional Resources:
- Pharma Assay Development
- Video: Tracing Blood Stem Cell Clones with Somatic Epimutations
- EBook: Single-Cell Sequencing 101










