The idea of “gene therapy” — genomic modification using exogenous DNA for therapeutic effect — was proposed long before the human genome was fully sequenced. Nevertheless, sequencing technologies have paradoxically reached a level of maturity not yet attained by cell and gene therapies (CGTs). Indeed, although next-generation sequencing (NGS) technologies have been around for over a decade, the first CGTs were approved by the FDA only a few years ago.
Now, advances in single-cell DNA sequencing technology promise to accelerate the development of CGTs by replacing conventional assays for analytical characterization with a single, high-throughput platform. This platform can be used to evaluate CGTs throughout the entire CGT pipeline, from protocol optimization to manufacturing and release testing.
What are Cell and Gene Therapies?
Cell and gene therapies (CGTs) provide a promising therapeutic avenue for the treatment of a wide variety of diseases, including cancer and inherited conditions. Not only do CGTs directly address the genetic root of these diseases, but they also offer the potential for durable, and even curative, outcomes.
This class of therapeutics aims to replace a faulty aspect of a patient’s biology. Gene therapies involve modifying the genome in vivo (within the body) in order to treat or cure a disease. Many gene therapies utilize viruses to deliver exogenous genes, as these vectors have a natural ability to enter cells that are otherwise hard to access. Depending on the virus used, the new gene may integrate into the genome or not. Gene therapies often function to replace a faulty gene with a healthy one so that normal proteins are restored.
Cell therapies entail introducing live cells into a patient to either treat or cure a disease. The cells are first collected ex vivo (outside the body) and may originate from the patient (autologous) or a donor (allogeneic). In some cases, cells may be genetically modified prior to expansion and infusion into the patient. These therapies are a combination of gene therapy and cell therapy.
A notable example of gene-modified cell therapies is chimeric antigen receptor T (CAR-T) cells — in which a patient’s T-cells are genetically modified so that they can better attack cancer cells. The T-cells are first extracted from the patient and genetically altered so they express synthetic molecules, called chimeric antigen receptors (CARs), on their surface. The CARs enable the cells to recognize a specific antigen on the surface of tumor cells so that they can better bind to and destroy them. After the T-cells are modified, they are expanded in the lab and subsequently reintroduced to the patient.
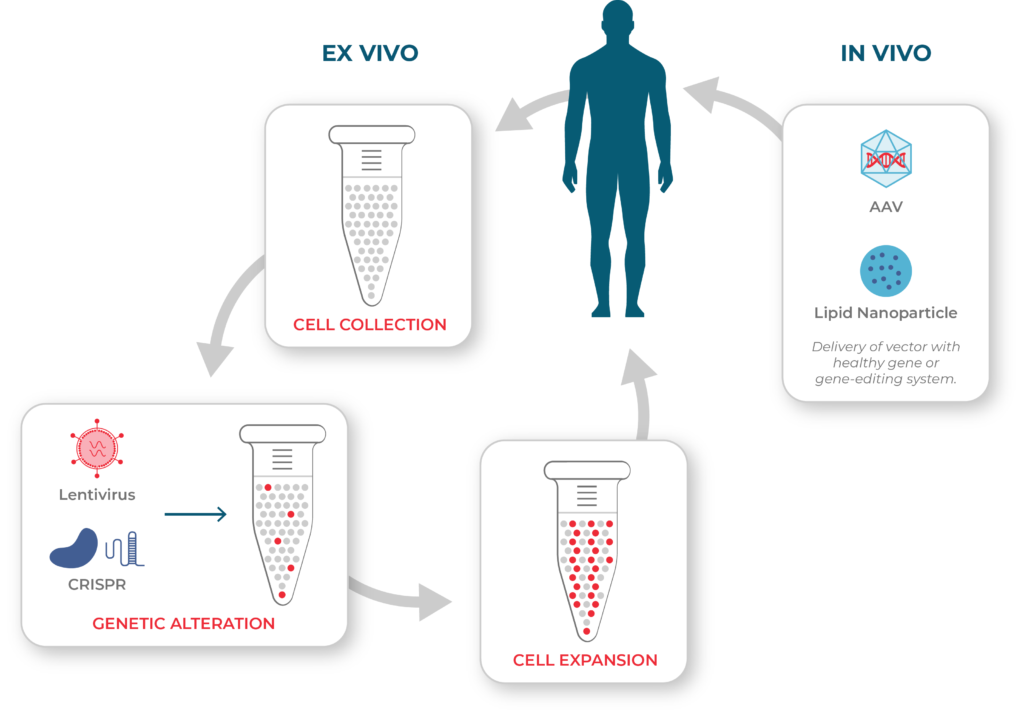
Cell and Gene Therapy Processes
Left: ex vivo cell therapies involve collecting cells from the patient or a donor. The cells may then be genetically modified before they are expanded and infused into the patient. Right: in vivo gene therapies are administered directly to the patient using a vector such as adeno-associated virus (AAV) or lipid nanoparticles.
CGT Development Is on the Rise
In recent years, the CGT field has blossomed with over 1,000 therapies being evaluated in clinical trials [1]. Among the diseases targeted in these trials are hemophilia, spinal muscular atrophy, Parkinson’s disease, inherited forms of blindness, and cancer. In 2017, the FDA approved CGTs targeting refractory blood cancers and an inherited form of retinal dystrophy [2].
The first experimental gene therapies used viral vectors to deliver and integrate exogenous DNA into the genome. However, the advent of nuclease-based gene-editing has expanded the scope of what’s possible in gene therapy by offering a new approach to genomic manipulation. For instance, clustered regularly interspaced short palindromic repeats (CRISPR) is a highly versatile tool that goes beyond a simple addition of genetic sequences. This tool also can be used to deactivate (knock out) or precisely change nucleotides. These broader capabilities present exciting new possibilities for CGT development. In fact, a CRISPR-mediated gene therapy for a rare disease, called transthyretin amyloidosis, has recently shown promising results in a clinical trial [3].
Changing the Genome is Not Always Perfect
As many researchers can attest, manipulating the genome can come with more than a modicum of variability. Although this is sometimes intentional, unwanted variation can hinder the development and production of CGTs. The impact of unwanted variation can be limited by understanding the factors that give rise to it. These factors differ depending on whether a virus or a gene editor is used for genomic manipulation.
Viruses can be used to integrate exogenous DNA into the genome, but doing so risks disrupting endogenous genes through random insertional mutagenesis. For example, viral integration can disrupt proto-oncogenes and lead to cellular transformation, a risk that increases with the number of viral integrations (i.e., viral copy number). On the other side of the coin, low viral transduction efficiency can limit the effectiveness of the therapy. These two factors — vector copy number and transduction efficiency — are important contributors to variability in virus-mediated CGT products.
The factors that contribute to variable gene editing outcomes are similar. Gene editors, like CRISPR or TALENs, can alter the wrong places in the genome (i.e., off-target effects). In addition, low on-target editing efficiency can hamstring a therapy the same way low viral transduction efficiency can. These factors — on- and off-target editing efficiency — contribute to variable gene editing outcomes.
Whether viral integrations or gene-editing tools are used, heterogeneity of CGT agents must be carefully measured and documented for regulatory approval. Measuring these factors during CGT development not only facilitates the optimization of CGTs, but also ensures that they are safe and work as expected in patients.
Characterizing CGTs is Challenging
Characterizing a CGT product is easier said than done. The simplest approach assays factors like copy number, transduction efficiency, or on-/off-target editing in bulk by sampling cells en masse after transduction or editing. However, bulk assays average these factors across an entire population of cells, obscuring any cell-to-cell variability that may exist. This lack of resolution can make it difficult to evaluate the manufacture, performance, and safety of a CGT product [2].
Isolating single-cell clones by limiting dilution represents a workaround that enables transduction/editing variability to be assessed with conventional assays like qPCR, ddPCR, and or NGS. This method, while not technically challenging, is very time-consuming. The process starts with the enrichment of positively transduced cells (using antibiotic or fluorescence-based selection) and is followed by a lengthy culturing period where individual clones are grown until there are sufficient cell numbers to evaluate each clonal line. Then, multiple assays are needed to fully evaluate each clone. The entire process can take months.
Single-cell DNA Sequencing Offers Solutions
Single-cell DNA sequencing (scDNA-seq) bypasses the limitations of traditional approaches. This approach can quantify CGT product specifications with single-cell resolution and without the need for clonal outgrowth. Moreover, scDNA-seq can assay multiple endpoints in a single assay. Mission Bio’s Tapestri Platform can evaluate up to 10,000 cells in a single sample — orders of magnitude higher than what is feasible with conventional methods. Simply put, scDNA-seq can considerably reduce the time and effort spent on CGT development.
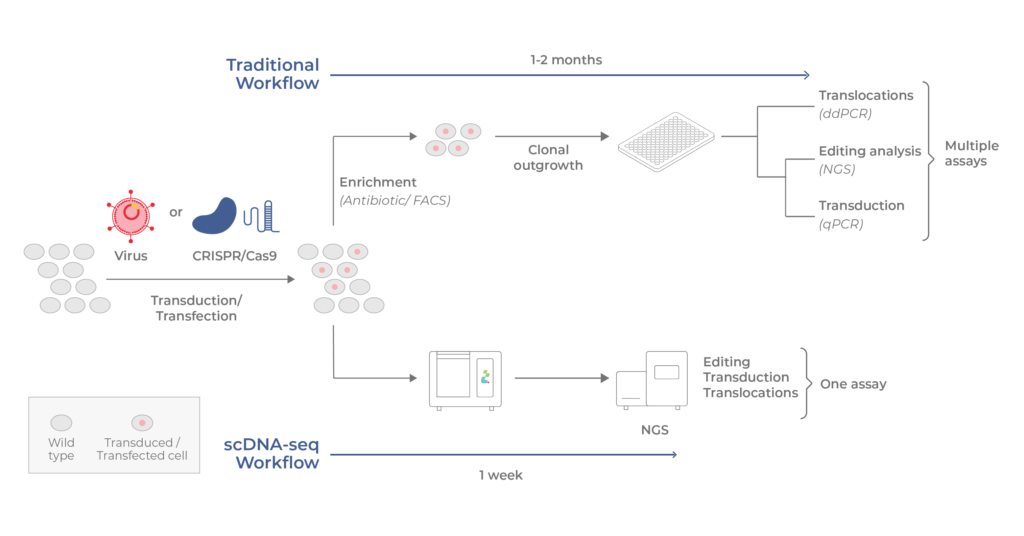
Traditional versus scDNA-seq workflows
Top: in the conventional workflow, transduced /transfected cells are enriched and clonally outgrown for several weeks. Assays that assess translocations and genome alterations are conducted separately. Bottom: with scDNA-seq using Tapestri, all analyses are combined in a single assay that can be completed in 1 week.
To summarize, as much as we are single-cell creatures at our foundation, CGTs are single-cell products. It only takes one genetic change in one cell to cause a disease like cancer. Similarly, the success of a CGT product depends on its performance at the single-cell level, as variability in a CGT product can adversely impact its safety and efficacy. Single-cell technologies like the Tapestri Platform can minimize this impact by providing true measurements for every cell in a therapeutic product with a single, high-throughput assay.
Mission Bio’s Pharma Assay Development Is Here for You!
Mission Bio is dedicated to partnering with biopharmas so that single-cell analysis can be seamlessly integrated into cell and gene therapy pipelines. Our Pharma Assay Development (PAD) service is here to help every step of the way. Mission Bio’s experts will enable you to leverage single-cell DNA sequencing and multi-omics in the analytical characterization of your cell and gene therapy products. We are committed to advancing the CGT field by helping you succeed!
Want to learn more? These application notes illustrate how the Tapestri Platform can be leveraged to characterize CGTs that have been genetically altered through viral integration and gene editing.
Check out this webinar on the application of single-cell tech in CGT development.
References
- Alliance for Regenerative Medicine Q2 2019: Quarterly Global Regenerative Medicine Sector Report. 2019 http://alliancerm.org/wp-content/uploads/2019/07/ARM_Q2_2019_Web_FINAL.pdf
- Dunbar, C.E. et al. Gene therapy comes of age. Science. 2018 Jan 12;359(6372):eaan4672.
- Ledford H. Landmark CRISPR trial shows promise against deadly disease. Nature. 2021 Jun 29.










